The lab has currently 4 established lines of research:
1- New mechanisms of autophagy relevant to neurodegeneration
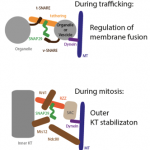 We explore the molecular requirements of autophagy and detail the function of novel factors in the process. For instance, we discovered Snap29, a new SNARE protein that specifically controls completion of autophagy (read about it). We have also found that Snap29 is likely controls kinetochore formation, ensuring appropriate spindle formation and execution of cell division (read about it). We are now investigating the role of Snap29 and other molecules associated to autophagy and proteostasis in neurodegeneration (read about it).
We explore the molecular requirements of autophagy and detail the function of novel factors in the process. For instance, we discovered Snap29, a new SNARE protein that specifically controls completion of autophagy (read about it). We have also found that Snap29 is likely controls kinetochore formation, ensuring appropriate spindle formation and execution of cell division (read about it). We are now investigating the role of Snap29 and other molecules associated to autophagy and proteostasis in neurodegeneration (read about it).
In the figure: Models of Snap29 activity in membrane fusion and kinetochore formation.
2- Regulation of Notch signaling
We untangled long-standing controversies concerning the regulation of Notch signaling by endocytosis, showing that in fly tissue the activating S3 cleavage of Notch occurs in acidic endosomal compartments and that the V-ATPase is a determinant of Notch activation (read about it). We also showed that V-ATPase is regulated to support Notch signaling during fly development, and that such process involves regulation of lysosome biogenesis and autophagy (read about it). We continue to identify and characterize new regulators of Notch signaling. The results collectively might inform therapeutic approaches to Notch- and V-ATPase-dependent tumors.
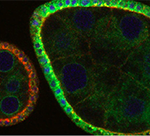
In the figure: Egg chambers of the Drosophila ovary stained to detect Notch (red) and expressing V-ATPase subunit c tagged with GFP (green). The cell nuclei are labeled in blue. Note the colocalization of Notch with V-ATPase in endosomes (yellow) in the follicular epithelium covering the germline.
3- Endo-lysosomal function, tissue development and tumorigenesis
We resuscitated the study of Drosophila tumor suppressor genes with the demonstration that ESCRT-mediated endosomal sorting is necessary to prevent tumorigenesis (read about it). We explored the functional differences between ESCRT complexes and showed that ESCRT-0 is involved in endosomal sorting but not in tumor suppression, highlighting a major difference in their activity (read about it). We continue to study aspects of this in fly models of brain tumors.

In the figure: Wild type Drosophila larvae (left) and chimeric larvae (right) carrying a ESCRT-mutant tumorous eye-antennal imaginal disc (right inset). Note the loss of epithelial morphology and overproliferation compared to the monolayer epithelium of a control eye-antennal disc (left inset).
4- Fly models of rare disease
We continue to use Drosophila and other simple models as avatars of patients affected by rare disease that cannot count on a lot of support for research. We have extensively investigated the pathogenesis of CEDNIK disease caused by loss of SNAP29 (read about it), we studied models of Cornelia De Lange syndrome (read about it), and we are providing insight relevant to congenital disorders of glycosylation (read about it) and lysosomal storage disorders. Stay tuned!
